Coronavirus Treatments
Background Information
A little info about the Coronavirus. It is related to the SARS outbreak in 2002, and is widely thought to have cold like symptoms, this was not true till the delta variant. Possibly 1/2 of patients seem relatively asymptomatic and are still infections. Symptoms to look for are lack of smell or taste, sore throat, headache, some nausea and diarrhea, as well as a cough. A lot have some chest pain that is intense, but not like the pressure of a heart attack. Late symptoms are high fever and trouble breathing. Below are some ideas of how to stay well as this threat continues. From Life Extension there is a very good review article about the origin of the Coronavirus and treatment options. They give recommendations for treatment and prevention. The full article can be found here @ Life Extension article.
Medications for Coronavirus
Here are some investigational uses of meds for treatments. However, your risk of acquiring Covid-19 is small if you follow the avoidance recommendations below. Those meds with best promise are given an α rating, those less likely a sequental Greek letter, those not much hope at all have a line trough them (these ratings change as evidence is available). There are also more available OTC meds following, as most listed investigational drugs wont be available even if ill! Photobiomodulation (PBM), is available at Landmark and uses your own natural stem cells to protect and heal.
[AAPS self at home treatment Hydroxychloroquine, Zinc, Ivermectin. Video by Dr. Peter McCullough & Dr. Elizabeth Lee Vliet. Possible investigational treatments Below]
I have moved this section up from the bottom of the page and no longer think the ideas so wild! Capricor, CAP-1002 (β) consists of allogeneic cardiosphere-derived cells, or CDCs, a unique population of cells that has been shown to exert potent immunomodulatory activity and alters the immune system’s activity to encourage cellular regeneration. CDCs have been the subject of over 100 peer-reviewed scientific publications and have been administered to approximately 140 human subjects across several clinical trials. CAP-1002 has been granted orphan drug designation by the FDA for the treatment of DMD (muscular dystrophy). The day Fauci was touting the 3.6% improvement of Remdesivir over control, this stock (CAPR), was up over 300% with 100% improvement (not the same kind of study, and future results may not be as good). Another company Pleuristem (β) has PLacental eXpanded (PLX) cells, placenta-derived, mesenchymal-like stromal cells, that are designed to be administered to patients without the need for tissue or genetic matching. They could be mass produced to stop the inflammation that kills most of these patients as well. Another company, Mesoblast, (γ)also uses allogeneic mesenchymal stem cells (MSCs), which play a crucial role supporting normal biological functions. MSCs secrete molecules that trigger blood vessel regeneration, repair tissues in the heart and brain, and moderate the immune system, similar to photobiomodulation. End of April, the treated patients on ventilator had a 80% survival versus the 80% death of non-treated (not a randomized control study though). ]
“Because there are no proven medical treatments for COVID-19 or other human coronaviruses, scientists are looking to both old and new antiviral drugs in search of effective therapies. Some, but not all, laboratory, animal, and preliminary human trials exploring the use of established antiviral medications against human coronaviruses have reported positive findings. This includes antiviral drugs used to treat human immunodeficiency virus (HIV) and hepatitis B and C, such as ribavirin (Ribasphere), lopinavir-ritonavir (Kaletra), and interferon beta-1b (Betaseron) (Sheahan 2020; Chu 2004; Dyall 2017, Lancet (γ)). The antimalarial drug chloroquine (Aralen, not high dose,+ZPack) has shown broad-spectrum antiviral effects in preclinical and clinical research, indicating its potential role in combined-drug approaches to treating emerging coronavirus infections (Dyall 2017)[In East Texas Chloroquine is not available, Hydroxychloroquine is limited and can only be prescribed in 14 day supply. It has to be used with a dx of Coronavirus so not prophylaxis. Zithromax is used with it and is in short supply in East TX as well. It can only be given with a dx as well. If you are a patient of Landmark schedule an appt and Dr. Baber can call a Rx in for you]. Preliminary evidence from multi-center clinical trials conducted in China suggest chloroquine phosphate has clinical efficacy against COVID-19 (Gao 2020).” Some more recent studies did not show benefit for Hydroxychloroquine, Kaletra or Arbidol in very sick patients (not available in US, Pub Mar 30, Li). Better news on the horizon with antibodies from Centivax and Dr. Glanville pub Mar 30.
“Remdesivir (β)is another antiviral drug that has shown promise against COVID-19. It is a prodrug of an adenosine analog that has potent antiviral activity against many RNA virus families (Agostini 2018). A 2018 in vitro study showed that remdesivir was efficacious against two strains of human endemic coronavirus (HCoV-OC43 and HCoV-229E) (Brown 2019). A drug screening study published on February 4th, 2020 showed remdesivir and chloroquine were both effective at inhibiting SARS-CoV-2 in vitro (Wang 2020). “
On Feb. 25th, 2020, the U. S. National Institutes of Health (NIH) announced the commencement of the first clinical trial of remdesivir for COVID-19 in the United States. Preliminary results (4/29/20) indicate that patients who received remdesivir had a 31% faster time to recovery than those who received placebo (p<0.001). Results also suggested a survival benefit, with a mortality rate by 14 days of 7.1% and 11.9% in the remdesivir and placebo groups, respectively.
NEW Another amazing drug leronlimab (α)from CytoDyn is having good results and works on the CCR5 receptor that is central to HIV infection but also a powerful modulator of inflammation. As of Apr 6, it has now been administered to 15 severely ill COVID-19 patients at four hospitals, under an emergency investigational new drug (EIND), which were granted by the FDA for each individual patient.
The issue of respiratory failure is probably one of the virus traveling along “a synapse‐connected route to the medullary cardiorespiratory center” from lung receptors. There are also ACE2 receptors in the brain which can be affected by hematogenous spread. The anosmia is evidence of neuro invasiviness and may affect cortical function and give a La belle indifference in regards to hypoxia. Coronavirusis have a “neuroinvasive propensity” and I believe is what cause so many deaths. It causes a hypo-ventilation relative to hypoxia effect. More normal ventilatory PEEP settings might be in order since it is a central issue, the brain, and not the lungs (MedScape,Cameron Kyle-Sidell, MD). The onset of infection to resp problems is about 2 weeks, and from symptoms onset to ICU about 8 days. Also, if you have Melatonin to take, it may just save your life in the late stages.
DPP-4 inhibitors (Januvia (Sitagliptin) Tradjenta) and GLP-1 analogs (Dulaglutide (Trulicity), Ozempic, Victoza, Byetta) has been associated with anti-inflammatory effects, which might be helpful in helping diabetes patients control COVID-19-related immune overreaction. MERS‐CoV enters human host cells mainly via dipeptidyl peptidase 4 (DPP4).
BTK, the oncogenic protein Calquence targets, is also a key regulator of the production of multiple inflammatory molecules in the lung. Therefore, BTK inhibition could theoretically reduce the release of these cytokines and dampen an excessive immune response. In real life, blood cancer patients who got BTK inhibitors also experienced decreased proinflammatory cytokines and chemokines.
SOLIRIS® (eculizumab) from Alexion who also makes ravulizumab, both are being used in ARDS, and some cases appear to be runaway complement activation from cytokine storm. These drugs work on the terminal portion of that cascade. Hopefully results with initiation of a clinical trial for Soliris.
Another choice tocilizumab (α, Actemra an interleukin-6 receptor antagonist( also IL-6 inhibitor from Sanofi Kevzara(γ)) that may prevent the Cytokine Release Syndrome, which may be causing the resp failure @ 8 mg/kg IV once; may be administered as alone or in combination with corticosteroids, If no clinical improvement in the signs and symptoms of CRS occurs after initial dose, may administer up to 3 additional doses; allow 8-hr interval between consecutive doses. Late use of steroids may save the patient contra Lancet. Roche clinical trial is underway COVACTA Aug 31. HYdroxychloroquine/Azithromycin/Tocilizumab trial TOCOVID Sept.
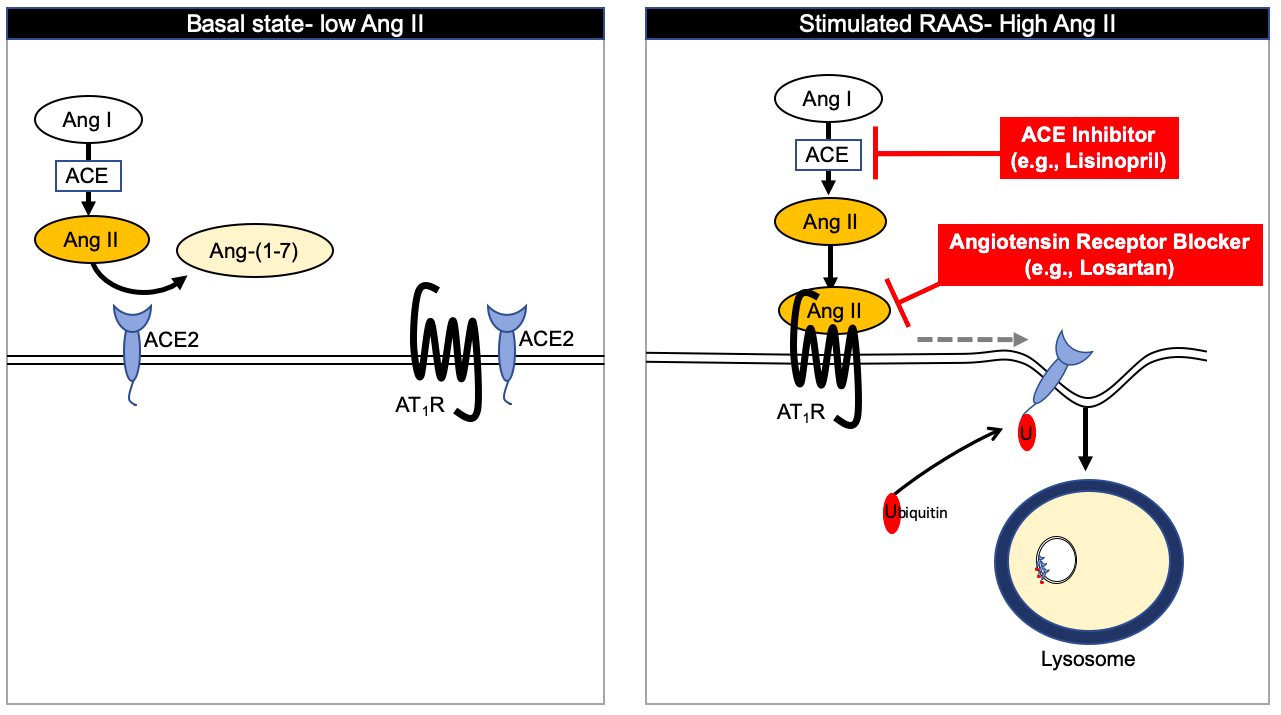
ACE2 Receptors
ACE/ARBs may have beneficial effect, (β, γ)and should not be stopped if you are on them. There is a very complicated explanation for why this may be so, but basically it involves blocking the ACE2 internalization and the Angiotensin1-7 product from ACE2. A treatment of acutely ill with Losartan, Kansas.
Hypothetical interactions Integrative Approaches COVID-19
Integrative Approaches COVID-19
There are many integrative therapies with well-established antiviral and immune-modulating properties. This is something you can do at home and follow the preventative measures at home. This virus is very much like a cold virus, but can cause problems in the elderly and those that have decreased immunity or lung conditions.
The interventions described in these protocols, though not necessarily validated as effective specifically for COVID-19, are nevertheless advisable upon onset of symptoms of upper respiratory tract infections.
For upper respiratory tract infections in general, including those caused by some types of Coronaviruses, Life Extension has long recommended swift action to help bolster your immune response and mitigate the likelihood of a severe disease course. At the first signs of an upper respiratory tract infection (eg, sneezing, coughing, feeling unwell, mild fever), make an appointment with your doctor then take the following:
- Zinc Lozenges : Completely dissolve in mouth one lozenge containing 18.75 mg of zinc acetate every two waking hours. Do not exceed 8 lozenges daily, and do not use for more than three consecutive days.
- Garlic: Take 9,000‒18,000 mg of a high-allicin garlic supplement each day until symptoms subside. Take with food to minimize stomach irritation.
- Vitamin D: If you do not already maintain a blood level of 25-hydroxyvitamin D over 50 ng/mL, then take 50,000 IU of vitamin D the first day and continue for three more days and slowly reduce the dose to around 5,000 IU of vitamin D each day. If you already take around 5,000 IU of vitamin D every day, then you probably do not need to increase your intake.
- Cimetidine: Take 800‒1,200 mg a day in divided doses. Cimetidine is a heartburn drug that has potent immune enhancing properties. (It is sold in pharmacies over-the-counter.)
- Melatonin: 3 to not more than 20 mg (higher doses may cause inflammation) at bedtime. Doses in the 10 mg range have shown great efficacy in delirium in ICU patients and a recent Italian study suggest even higher doses may help the immune response that is killing Covid-19 patients. Melatonin can boost your white blood cells involved in fighting the infection, can reduce the inflammatory protiens or cytokines that cause the inflammation that kills, and decrease the oxidative stress that also leads to cell death (excellent article Acute Lung Injury/Acute Resp Distress Syndrome ALI/ARDS ScienceDirect).

Do not delay implementing the above regimen. Once viruses that cause respiratory infections infect too many cells, they replicate out of control and strategies like zinc lozenges will not be effective. Treatment must be initiated as soon as symptoms manifest. Really, many of the treatments are best started weeks in advance to get the immune and anti-inflammatory benefits. Although this regimen has not been studied specifically in the context of COVID-19, there is little reason not to implement this strategy as there is a reluctance to use anything not tested by high quality studies in the medical/political environment which thus entails a high cost to use .
Below are a few additional integrative interventions that have shown beneficial immune-enhancing effects in the context of viral upper respiratory tract infections.
- Vitamin C. Several studies have shown that vitamin C supplementation, both before and soon after the onset of symptoms of upper respiratory tract infections, may help ease symptom burden and reduce the duration of illness (Gorton 1999; Hemilä 1999; Ran 2018). However, the available evidence does not consistently support the notion that preventive vitamin C supplementation can reduce the risk of acquiring upper respiratory tract infections (Hemilä 2013; Virilhon 2019). Importantly, studies to date have not focused specifically on coronavirus infections but on upper respiratory tract infections in general such as those caused by rhinoviruses, enteroviruses, and influenza viruses.
As of March 4th, 2020, a study is slated to take place in Wuhan, China to test the effects of 24-gram intravenous vitamin C infusions on outcomes in COVID-19 patients. The primary outcome will assess ventilation-free days, and one of several secondary outcomes will be 28-day mortality (Peng 2020). Previously, a 2017 case report suggested that high-dose intravenous vitamin C may have contributed to the recovery of a 20-year-old patient with acute respiratory distress syndrome (ARDS) due to a viral respiratory tract infection (Fowler 2017).
- Lactoferrin. Lactoferrin is a glycoprotein involved in immune response and several other functions (Giansanti). It is found in secreted fluids and is abundant in milk (breast and cow). Lactoferrin has well-documented antibacterial, antiviral, and antifungal properties (Malaczewska 2019; Wakabayashi 2014; Ishikawa 2013). It appears to exert antiviral effects by activating the antiviral cytokines interferon (IFN)-α/β and boosting natural killer (NK) cell activity and Th1 cytokine responses (Wakabayashi 2014). Some studies suggest that lactoferrin administration may reduce the incidence and severity of common respiratory tract viral infections, like colds and flu (Vitetta 2013; Wakabayashi 2014).
In 2005, researchers reported that the gene encoding lactoferrin was highly upregulated in patients affected during the SARS epidemic that emerged in 2003, suggesting that it plays a role in the innate immune response to the infection (Reghunathan 2005). A follow-up study indicated that lactoferrin prevented the 2003 SARS coronavirus from entering host cells (Lang 2011 Heparin maybe helpful in the hospital). No data have been published as of March 10th, 2020 directly linking lactoferrin with outcomes in COVID-19 patients.
- Selenium. Selenium has important antioxidant, anti-inflammatory, and antiviral activities in the body, and deficiency is associated with increased risk of viral infection (Wrobel 2016). In patients with HIV infection, poor selenium status is correlated with increased mortality, and supplementation has been reported to slow progression of immune dysfunction and reduce hospital admissions (Wrobel 2016; Muzembo 2019). Some researchers have proposed that lack of selenium in regional soils may have contributed to the SARS outbreak in 2003 (Harthill 2011).
- Probiotics. A growing body of evidence shows probiotic supplements with Bifidobacterium and Lactobacillus species can enhance antiviral immune activity and may reduce the occurrence, severity, and duration of viral respiratory tract infections such as influenza (Lenoir-Wijnkoop 2019; Mousa 2017).
- Epigallocatechin gallate (EGCG). EGCG is a polyphenol from green tea. Because of its broad antiviral effects, EGCG has been proposed as a promising agent for preventing and treating viral infections such as SARS and MERS (Kaihatsu 2018; Hsu 2015, Chen Black tea and pur maybe better at blocking Coronavirus entry).
Protective Measures Coronavirus
Below are some basic measures to consider in order to reduce your risk of contracting COVID-19 and other viral illnesses.
-
Avoid air travel to affected regions. Avoiding contact with infected individuals is the best way to protect yourself from COVID-19. Since most of the cases are occurring in China at this time, the United States Centers for Disease Control and Prevention (CDC) has issued a travel warning that recommends avoiding non-essential travel to China (CDC 2020c).
In addition, all air travel is associated with exposure to people and the infectious agents they carry. Outbreaks of infectious illnesses, including measles, influenza, SARS, and many others, aboard commercial flights have been documented (Mangili 2015; Hertzberg 2016). Therefore, avoiding air travel is a reasonable precaution for reducing your risk of viral infections in general, particularly if you have other vulnerabilities.
- Wash your hands. Frequent hand washing is an important strategy for protecting against all types of infectious diseases. Corona viruses have a lipid protective coat and soap and warm water break this down quickly. Studies in office and healthcare settings have further demonstrated strategic use of alcohol-based surface disinfectants and hand sanitizers can reduce viral spread by 85–94% (Kurgat 2019; Reynolds 2019). See PDF below for healthcare worker safety.
- Strengthen immunity. Optimal functioning of the immune system is vital for defending against all types of infections, from mild colds to dangerous influenza and life-threatening pneumonia. A nutrient-dense diet, regular exercise, adequate sleep, and stress management can all contribute to healthy immune function (Zapatera 2015).
- Disinfect surfaces. Coronaviruses can persist on inanimate surfaces like metal, glass, or plastic for up to nine days. Fortunately, coronaviruses can be inactivated with proper cleaning and disinfecting agents. Therefore, keeping surfaces clean and properly disinfected is important to limit the spread of infectious diseases caused by coronaviruses. A study published on February 6th, 2020 found that coronaviruses on inanimate surfaces can be inactivated within one minute through disinfection with 62%‒71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite (eg, bleach) (Kampf 2020).
As of March 6th, 2020, the United States Environmental Protection Agency (EPA) provides a list of EPA-registered disinfectant products for use against the SARS-CoV-2 virus (EPA 2020). The list is available on the EPA’s web page, here: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2.
-
Social distancing. The CDC and other health authorities worldwide strongly advise that citizens—especially those at increased risk—living in communities experiencing community spread of COVID-19 “(remain) out of congregate settings, avoiding mass gatherings, and maintaining distance (approximately 6 feet or 2 meters) from others when possible” (CDC 2020e).
- Shoes Outside. In Hawaii we take our shoes off outside, and due to the nature of the Coronavirus falling with gravity, areas of high traffic will be bad areas to pick up the virus on your shoe. If you walk through sand this may clean to some extent, but there will still be virus that may have virus on the sides. So treat them as contaminated after you have been through stores, hospital ERs or areas with high foot traffic. Wash your hand after you take the shoes off!

“Most people who catch Covid-19—the disease caused by the novel coronavirus—start showing symptoms roughly five days after infection,” according disease analysts at Johns Hopkins.
“Other researchers studying a smaller number of cases estimated Covid-19 has an incubation period as short as two days to as long as 14 days, with a few reported cases taking up to 27 days to develop.”
“In research published Monday online in the academic medical journal Annals of Internal Medicine, the scientists calculated that the median incubation period of the virus is 5.1 days. All told, about 97.5% of those who develop symptoms will do so within 11.5 days of exposure, the scientists said.”
Even so, they cautioned that, as a matter of statistical probability, the 14-day monitoring and quarantine period likely would miss some cases. By their calculations, they estimated that for every 10,000 people quarantined for 14 days, slightly more than 100 or so would develop symptoms only after being released from isolation.
www.wsj.com/amp/articles/coronavirus-sym…ch-finds-11583784452
ICU_one_pager_COVID_v2.6
Coronavirus-COVID-1
COVID-19-Guidelines-for-Staff-Safety-4-9-FINAL
Proposed-Covid-Tx
Ways to sterilize masks. https://www.medscape.com/viewarticle/928877 NEPHJC position ACE/ARB http://www.nephjc.com/news/covidace2 Dr Weingart in the ER/ICU trenches https://www.hippoed.com/em/ercast/episode/tbd3/covid19weingart
Skin Rashes
Of the 88 COVID-19 patients, 20.5% developed skin manifestations. Eight of the 18 (44%) had skin eruptions at symptom onset, and the rest after hospitalization. Fourteen (78%) had red rashes, three had widespread urticaria, and one had chickenpox-like vesicles. The most commonly affected area was the trunk. Itching was mild or absent, and lesions usually healed up in a few days. Most importantly, skin manifestations did not correlate with disease severity. These skin manifestations “are similar to cutaneous involvement occurring during common viral infections,” said the author of the report, Sebastiano Recalcati, MD, a dermatologist at Alessandro Manzoni Hospital. Dermatolgy News
Elderly Presentations
If risk of exposure then symptoms can be minimal in the elderly. Seniors may seem “off” – not acting like themselves – early on after being infected by the coronavirus. They may sleep more than usual or stop eating. They may seem unusually apathetic or confused, losing orientation to their surroundings. They may become dizzy and fall. Sometimes, seniors stop speaking or simply collapse (MDEdge).
Pediatric Kawasaki’s Disease
There are post inflammatory responses that occur in young children after viral illnesses that have a constellation of symptoms consistent with Kawasaki’s disease. Other varieties of coronavirus have been implicated in Kawasaki’s disease, and it is no surprise that Sars-Cov would be as well. Some of the viral titers may be negative, but auntie Patty tigers are positive, but not in all cases.
Neurologic manifestations
Awareness of the possible neuroinvasion may have a guiding significance for the prevention and treatment of the SARS‐CoV‐2‐induced respiratory failure (Wiley). COVID-19 symptoms may also could include subtle neurologic deficits, severe fatigue, trigeminal neuralgia, complete/severe anosmia, myalgia, later and more progressive symptoms like encephalopathy, ataxia, even 1 case of necrotizing hemorrhagic encephalopathy, altered levels of consciousness and later Guillain-Barre syndrome (MedScape)
Cardiac Presentations
Presentations can be ST seg elevation in MI, cardiogenic shock in patients with acute systolic heart failure and viral Covid 19 illness in transplant. MDEdge

SARS-CoV-2 Mutations
Viral mutations rate may have signs of decreased pathogenicity, but not much. You can track the viral mutation at LANL in Los Alamos (video). Also, the linked paper has graphs of the dominate ?G419 mutation.
We show for the fist time that ferritin activates an iron-independent signaling cascade involving PI3-kinase, PKCζ, MEK-1/2 and p44/p42 MAP-kinase resulting in the activation of p50/p65 NFκB and the significantly enhanced expression of hepatic proinflammatory and profibrogenic mediators IL-1β, iNOS, RANTES, IκBα and ICAM1. Rudell 2009
Recent Wild Ideas
Also myokine 6 is the reason CoolTone has immunological benefits. CoolTone and the idea of using magnetic fields to induce muscle contractions is relatively new, and the mechanism of hypertrophy is not through muscle breakdown, but I believe biostimulation.
Out of Stanford, using CRISPR gene splicing technology, they may be able to treat H1N1 and CV19 with the same treatment! It involves using CAS13 to cleave the viral RNA and knock the viral load down to 90%. They are working on the delivery mechanism, lipopeptides, to get the CAS13 inside the infected lung cells.


At a time of unprecedented panic over the rapidly spreading COVID-19 coronavirus, several research groups are asking whether existing therapies could be repurposed in fighting the disease. And pretty much everything seems to be on the table, including melatonin, the hormone that’s sold over-the-counter as a sleep aid.
The melatonin suggestion was published Monday in the journal Cell Discovery by Cleveland Clinic researchers, who hit on the hormone after analyzing the genomes of 15 human coronaviruses. The team compared COVID-19 with the coronavirus that caused the SARS outbreak of 2003 using a technique called “network proximity analysis” to identify combinations of existing drugs that may be able to target cellular factors that allow the viruses to replicate.
The analysis turned up three potential drug combinations: melatonin plus chemotherapy drug mercaptopurine; sirolimus, most commonly used to prevent organ-transplant rejection, combined with another chemo drug, dactinomycin; and breast cancer drug toremifene plus emodin, a chemical found in plants like rhubarb.
https://www.fiercebiotech.com/research/melatonin-stem-cells-researchers-step-up-unconventional-approaches-to-covid-19?mkt_tok=eyJpIjoiWmpnNFl6UmtZakF5TnpabCIsInQiOiI1XC95dVlIa1AwSjF5Zm1KNlduc2h2MWd4YzdCTXpLU2tma2tvV0k3RUM4enhPaWw4ZFNyc3ZQQXJNTEN2YWRHUEN4bk9MR3dcL3dOT0wwNkMxOWJFUFVsbXFpdFh4MzBzUkY3c1pkelFtT0UrK1NHOFh4UysrOXI1TWxFTmRVbGhnIn0%3D&mrkid=9160999
The genome of SARS-CoV-2 shares about 80% identity with that of SARS-CoV and is about 96% identical to the bat coronavirus BatCoV RaTG13
for the structure of the Coronovirus protein interaction with ACE2 receptor see link
https://science.sciencemag.org/content/early/2020/03/03/science.abb2762
COVID-19 is an emerging, rapidly evolving situation.
Get the latest public health information from CDC: https://www.coronavirus.gov .
Get the latest research from NIH: https://www.nih.gov/coronavirus.
Latest map from John Hopkins https://coronavirus.jhu.edu/map.html
Malaria drug chloroquine, AbbVie’s HIV combo therapy Kaletra and an influenza med called Arbidol are among top candidates that physicians are repurposing for the treatment of COVID-19. Despite backing from officials, though, the three have disappointed separately in two Chinese clinical trials of mild patients.
Hydroxychloroquine, a more tolerable form of chloroquine, didn’t top placebo at clearing the coronavirus among mild Chinese patients, or helping them reach normal temperature sooner, Evercore ISI analyst Umer Raffat noted in a Tuesday memo.
Separately, neither Kaletra—a combination of HIV antivirals lopinavir and ritonavir—nor Arbidol (umifenovir) delivered benefits in viral clearance or symptom relief compared with no antiviral treatment in a small Chinese study in mild-to-moderate COVID-19 patients, results published Monday on the preprint site medRxiv show.
Chinese health authorities first included Kaletra in their coronavirus treatment guidelines in late January, and chloroquine and Arbidol—which is available in China and Russia, but not in Western countries—were added in an update on Feb. 19. These decisions were backed by some early preclinical evidence in lab dishes or anecdotal success in the clinic amid a lack of approved drugs for the new pathogen. During a press conference on Thursday, President Donald Trump highlighted chloroquine as a promising candidate.
But so far, these three meds have not lived up to their expectations in clinical trials.
https://www.fiercebiotech.com/biotech/biopharma-s-no-holds-barred-fight-to-find-a-covid-19-vaccine-full-list
apologize to the grammar and spelling police. This one will not be proofread and much shorter.
I am an ER MD in New Orleans. Class of 98. Every one of my colleagues have now seen several hundred Covid 19 patients and this is what I think I know.
Clinical course is predictable.
2-11 days after exposure (day 5 on average) flu like symptoms start. Common are fever, headache, dry cough, myalgias(back pain), nausea without vomiting, abdominal discomfort with some diarrhea, loss of smell, anorexia, fatigue.
Day 5 of symptoms- increased SOB, and bilateral viral pneumonia from direct viral damage to lung parenchyma.
Day 10- Cytokine storm leading to acute ARDS and multiorgan failure. You can literally watch it happen in a matter of hours.
81% mild symptoms, 14% severe symptoms requiring hospitalization, 5% critical.
Patient presentation is varied. Patients are coming in hypoxic (even 75%) without dyspnea. I have seen Covid patients present with encephalopathy, renal failure from dehydration, DKA. I have seen the bilateral interstitial pneumonia on the xray of the asymptomatic shoulder dislocation or on the CT’s of the (respiratory) asymptomatic polytrauma patient. Essentially if they are in my ER, they have it. Seen three positive flu swabs in 2 weeks and all three had Covid 19 as well. Somehow this ***** has told all other disease processes to get out of town.
China reported 15% cardiac involvement. I have seen covid 19 patients present with myocarditis, pericarditis, new onset CHF and new onset atrial fibrillation. I still order a troponin, but no cardiologist will treat no matter what the number in a suspected Covid 19 patient. Even our non covid 19 STEMIs at all of our facilities are getting TPA in the ED and rescue PCI at 60 minutes only if TPA fails.
Diagnostic
CXR- bilateral interstitial pneumonia (anecdotally starts most often in the RLL so bilateral on CXR is not required). The hypoxia does not correlate with the CXR findings. Their lungs do not sound bad. Keep your stethoscope in your pocket and evaluate with your eyes and pulse ox.
Labs- WBC low, Lymphocytes low, platelets lower then their normal, Procalcitonin normal in 95%
CRP and Ferritin elevated most often. CPK, D-Dimer, LDH, Alk Phos/AST/ALT commonly elevated.
Notice D-Dimer- I would be very careful about CT PE these patients for their hypoxia. The patients receiving IV contrast are going into renal failure and on the vent sooner.
Basically, if you have a bilateral pneumonia with normal to low WBC, lymphopenia, normal procalcitonin, elevated CRP and ferritin- you have covid-19 and do not need a nasal swab to tell you that.
A ratio of absolute neutrophil count to absolute lymphocyte count greater than 3.5 may be the highest predictor of poor outcome. the UK is automatically intubating these patients for expected outcomes regardless of their clinical presentation.
An elevated Interleukin-6 (IL6) is an indicator of their cytokine storm. If this is elevated watch these patients closely with both eyes.
Other factors that appear to be predictive of poor outcomes are thrombocytopenia and LFTs 5x upper limit of normal.
Disposition
I had never discharged multifocal pneumonia before. Now I personally do it 12-15 times a shift. 2 weeks ago we were admitting anyone who needed supplemental oxygen. Now we are discharging with oxygen if the patient is comfortable and oxygenating above 92% on nasal cannula. We have contracted with a company that sends a paramedic to their home twice daily to check on them and record a pulse ox. We know many of these patients will bounce back but if it saves a bed for a day we have accomplished something. Obviously we are fearful some won’t make it back.
We are a small community hospital. Our 22 bed ICU and now a 4 bed Endoscopy suite are all Covid 19. All of these patients are intubated except one. 75% of our floor beds have been cohorted into covid 19 wards and are full. We are averaging 4 rescue intubations a day on the floor. We now have 9 vented patients in our ER transferred down from the floor after intubation.
Luckily we are part of a larger hospital group. Our main teaching hospital repurposed space to open 50 new Covid 19 ICU beds this past Sunday so these numbers are with significant decompression. Today those 50 beds are full. They are opening 30 more by Friday. But even with the “lockdown”, our AI models are expecting a 200-400% increase in covid 19 patients by 4/4/2020.
Treatment
Supportive
worldwide 86% of covid 19 patients that go on a vent die. Seattle reporting 70%. Our hospital has had 5 deaths and one patient who was extubated. Extubation happens on day 10 per the Chinese and day 11 per Seattle.
Plaquenil which has weak ACE2 blockade doesn’t appear to be a savior of any kind in our patient population. Theoretically, it may have some prophylactic properties but so far it is difficult to see the benefit to our hospitalized patients, but we are using it and the studies will tell. With Plaquenil’s potential QT prolongation and liver toxic effects (both particularly problematic in covid 19 patients), I am not longer selectively prescribing this medication as I stated on a previous post.
We are also using Azithromycin, but are intermittently running out of IV.
Do not give these patient’s standard sepsis fluid resuscitation. Be very judicious with the fluids as it hastens their respiratory decompensation. Outside the DKA and renal failure dehydration, leave them dry.
Proning vented patients significantly helps oxygenation. Even self proning the ones on nasal cannula helps.
Vent settings- Usual ARDS stuff, low volume, permissive hypercapnia, etc. Except for Peep of 5 will not do. Start at 14 and you may go up to 25 if needed.
Do not use Bipap- it does not work well and is a significant exposure risk with high levels of aerosolized virus to you and your staff. Even after a cough or sneeze this virus can aerosolize up to 3 hours.
The same goes for nebulizer treatments. Use MDI. you can give 8-10 puffs at one time of an albuterol MDI. Use only if wheezing which isn’t often with covid 19. If you have to give a nebulizer must be in a negative pressure room; and if you can, instruct the patient on how to start it after you leave the room.
Do not use steroids, it makes this worse. Push out to your urgent cares to stop their usual practice of steroid shots for their URI/bronchitis.
We are currently out of Versed, Fentanyl, and intermittently Propofol. Get the dosing of Precedex and Nimbex back in your heads.
One of my colleagues who is a 31 yo old female who graduated residency last may with no health problems and normal BMI is out with the symptoms and an SaO2 of 92%. She will be the first of many.
I PPE best I have. I do wear a MaxAir PAPR the entire shift. I do not take it off to eat or drink during the shift. I undress in the garage and go straight to the shower. My wife and kids fled to her parents outside Hattiesburg. The stress and exposure at work coupled with the isolation at home is trying. But everyone is going through something right now. Everyone is scared; patients and employees. But we are the leaders of that emergency room. Be nice to your nurses and staff. Show by example how to tackle this crisis head on. Good luck to us all.
***************************************************************************************************************************
In uncertain times, we search for facts to provide comfort and clarity. Throw in the power of Texags.com and I should not be so dumbfounded by the run this is getting.
My first expanded lost draft (thanks again MacBook Touch Bar) contained the appropriate hedging, disclaimers, and uncertainty the current understanding of this pandemic deserves. Some of the more concise, unproofread, hastily rewritten original post (OP) presents itself as more definitive instead of “what I think I know”. For this, my apologies. I am not performing clinical trials. I am not involved in cohorting and analyzing data. The academic physicians involved in ER, Infectious Disease and Pulmonary Critical Care are likely (hopefully) way beyond my understanding of Covid 19. Furthermore, I fail to appreciate any additional benefit I could provide to Hospital Administrators who have been preparing and communicating with each other for months; or for some already combating this daily.
Basically, several state medical licensing boards are temporarily loosening their independent practice regulations on NPs, PAs, and to a lesser extent Medical Students. I wrote the OP as much for me to collect and organize my thoughts, as it was to provide a jumping-off platform for providers who may find themselves in an ER-like setting and unknowingly be treating Covid 19 patients or will be treating them soon enough. If any of it helps some of my colleagues hit the ground running then that is something too.
The OP was a summary of thoughts from being emersed in Covid 19 for weeks, reading as much as I can, and following up on my own patients. It is not my intention for this to be taken as dogma. I do not have the answers or the algorithm everyone is searching for. I was merely looking to point the handful of people I thought would read it in a clinical direction best I could. What I think I know evolves every day with the presumption it may very well end up markedly different once this pandemic is better understood. I do not know when this crisis will ultimately be arrested, however, I maintain resolute that each one of us can help make that happen. Stay home. Be safe. Find a way not to have to visit grandma.
Thank you to all the well-wishers and good luck to us all.
Sincerely,
https://texags.com/forums/84/topics/3102444
Pharyngitis (red throat without exudate) may be a symptom of mild illness. Over half of patient on a cruise ship from Japan that tested positive were asymptomatic. 705 COVID-19 cases were confirmed among 4,061 passengers/crew (17 % infected after a month in close contact): 392 cases were asymptomatic( 56%) : 36 persons were admitted to intensive care units (5% of infected assuming the 6 deaths were of ICU, <0.9% of exposed) : and 6 patients died (0.9% 0r 0.1% of exposed). https://wwwnc.cdc.gov/eid/article/26/6/20-0452_article
The initial spread was max 2.8 (or for every infected pt almost 3 would get infected), it seemed to be mostly one deck on the Diamond Princess and those involved in food processing as they were in close quarters. https://www.cdc.gov/mmwr/volumes/69/wr/mm6911e2.htm?s_cid=mm6911e2_w
[…] indicate sufficient energy. Alternate, treatment could be over carotids and sub auricular. If the La Belle Indifferance is really supracortical, then treatment of the frontal area may suffice. This would limit viral […]
The World Health Organization and a number of national governments have changed their Covid-19 policies and treatments on the basis of flawed data from a little-known US healthcare analytics company, also calling into question the integrity of key studies published in some of the world’s most prestigious medical journals.
A Guardian investigation can reveal the US-based company Surgisphere, whose handful of employees appear to include a science fiction writer and an adult-content model, has provided data for multiple studies on Covid-19 co-authored by its chief executive, but has so far failed to adequately explain its data or methodology.
Data it claims to have legitimately obtained from more than a thousand hospitals worldwide formed the basis of scientific articles that have led to changes in Covid-19 treatment policies in Latin American countries. It was also behind a decision by the WHO and research institutes around the world to halt trials of the controversial drug hydroxychloroquine. On Wednesday, the WHO announced those trials would now resume.
Two of the world’s leading medical journals – the Lancet and the New England Journal of Medicine – published studies based on Surgisphere data. The studies were co-authored by the firm’s chief executive, Sapan Desai.
Late on Tuesday, after being approached by the Guardian, the Lancet released an “expression of concern” about its published study. The New England Journal of Medicine has also issued a similar notice.
An independent audit of the provenance and validity of the data has now been commissioned by the authors not affiliated with Surgisphere because of “concerns that have been raised about the reliability of the database”.
Questions raised over hydroxychloroquine study which caused WHO to halt trials for Covid-19
Read more
The Guardian’s investigation has found:
A search of publicly available material suggests several of Surgisphere’s employees have little or no data or scientific background. An employee listed as a science editor appears to be a science fiction author and fantasy artist https://www.theguardian.com/world/2020/jun/03/covid-19-surgisphere-who-world-health-organization-hydroxychloroquine
Azithromycin how it may work for CV19 and help prevent aging!
https://www.aging-us.com/article/101633/text
Potential role of autophagy in conferring “senolytic” activity. Autophagic cells have an increased tendency to become senescent. Mechanistically, autophagic cells accumulate large numbers of lysosomes and auto-phagosomes. These organelles contain high levels of proteases, such as cathepsins (B, S and L). Interestingly, it has been previously demonstrated that lysosomes in authophagic cells can become “leaky” due to an acute stress, ultimately resulting in stress-induced senescence (SIS). As a consequence, cathepsins leak into the cytoplasm where they can then cleave sirtuin family members (e.g., SIRT1), paving the way for the onset of senescence. Here, we show that a weak autophagy inducer, Azithromycin (AZ), selectively targets senescent cells. We speculate that the weak induction of autophagy in senescent cells can result in cell death.